¶ Humanin
Humanin (HN) is a 24-amino acid peptide encoded by the mitochondrial 16S ribosomal RNA (MT-RNR2) gene. It was the first identified member of a class of bioactive peptides known as mitochondrial-derived peptides (MDPs). Discovered in 2001 in the context of Alzheimer's disease research, Humanin is characterized by its potent cytoprotective effects, particularly its ability to suppress apoptosis (programmed cell death) and protect cells from oxidative stress and mitochondrial dysfunction.
While naturally produced in the body, Humanin levels decline progressively with age, a trend that is reversed in some centenarian populations, suggesting a link to exceptional longevity.
¶ Evidence Summary
- Evidence Grade: Promising (Yellow)
- Primary Benefits: Neuroprotection, cytoprotection, metabolic regulation.
- Key Mechanism: Inhibition of Bax-mediated apoptosis; activation of STAT3 and ERK signaling pathways.
- Current Status: Extensive preclinical evidence (cell/animal); observational human data; no approved therapeutic drugs.
¶ Biological Origin and Structure
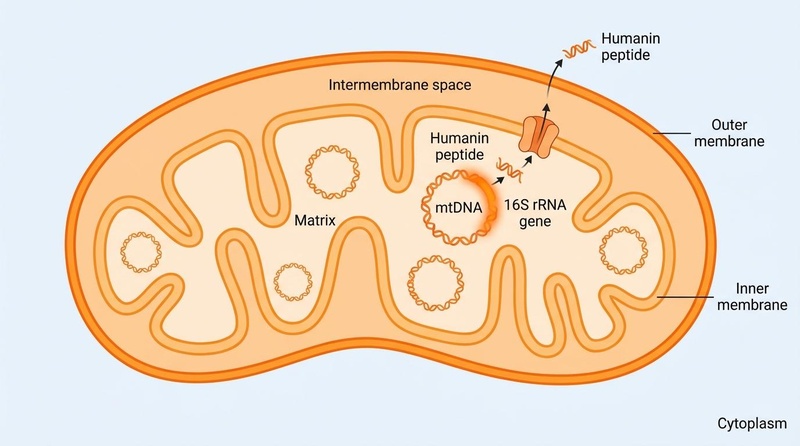
Unlike most peptides which are encoded by nuclear DNA, Humanin is encoded within the mitochondrial genome. This unique origin classifies it as a "mitokine"—a signaling molecule derived from mitochondria that communicates cellular stress status to the rest of the organism.
- Sequence: The standard secreted form consists of 24 amino acids:
Met-Ala-Pro-Arg-Gly-Phe-Ser-Cys-Leu-Leu-Leu-Leu-Thr-Ser-Glu-Ile-Asp-Leu-Pro-Val-Lys-Arg-Arg-Ala. - Analogs: To improve stability and potency, a synthetic analog known as HNG (S14G-Humanin) was developed by replacing the serine at position 14 with glycine. HNG is approximately 1,000 times more potent than native Humanin in cytoprotective assays[1][2].
¶ Mechanisms of Action
Humanin exerts its protective effects through a dual mechanism: intracellular inhibition of cell death machinery and extracellular receptor-mediated signaling.
¶ Intracellular: "The Molecular Shield"
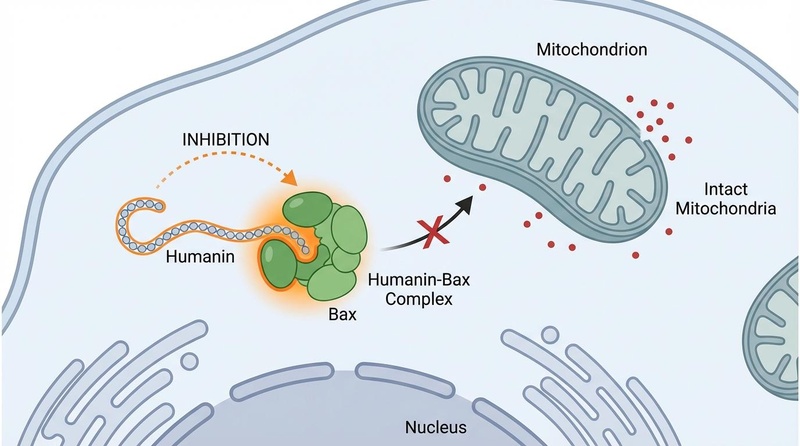
Inside the cell, Humanin interacts directly with pro-apoptotic proteins of the Bcl-2 family, acting as a brake on programmed cell death.
- Bax Inhibition: Upon cellular stress, the protein Bax translocates to the mitochondria to initiate cell death. Humanin binds to Bax, preventing this translocation and maintaining mitochondrial integrity[3][4].
- IGFBP-3 Inhibition: Humanin binds to Insulin-like Growth Factor Binding Protein-3 (IGFBP-3), blocking its ability to induce apoptosis and potentially modulating IGF-1 availability[5].
¶ Extracellular: Receptor Signaling
Secreted Humanin acts as an autocrine and paracrine signal by binding to specific cell-surface receptors:
- FPR2 Receptor: Binding to Formyl Peptide Receptor 2 (FPR2) activates the ERK1/2 signaling pathway, which is associated with cell survival and reduced inflammation[6].
- Trimeric Receptor Complex: Humanin binds to a complex composed of CNTFR, WSX-1, and gp130 subunits. This activates the JAK2/STAT3 pathway, critical for neuroprotection and insulin sensitivity[7].
¶ Neuroprotective Effects
Humanin was originally discovered for its ability to rescue neurons from toxicity associated with Alzheimer's disease (AD).
¶ Alzheimer's Disease and Amyloid Beta
Preclinical models demonstrate that Humanin can mitigate the toxicity of amyloid-beta (A) plaques, a hallmark of AD.
- Mechanism: Humanin competes with A for binding to the FPR2 receptor, blocking the neurotoxic signals triggered by amyloid accumulation. It also prevents A-induced synaptic loss and dendritic degeneration[8][9].
- Evidence: In triple-transgenic AD mice (3xTg-AD), treatment with the HNG analog reduced A plaque burden and improved cognitive function[10].
¶ Stroke and Ischemia
Humanin has shown efficacy in reducing brain injury following stroke. In mouse models of middle cerebral artery occlusion (MCAO), HNG pretreatment reduced infarct volume by approximately 50%, preserving brain tissue through anti-apoptotic pathways[11].
- Certainty: Moderate (High quality animal data; lack of human interventional trials).
¶ Metabolic and Cardiovascular Health
Humanin plays a significant role in regulating metabolism and protecting the cardiovascular system from aging-related damage.
¶ Atherosclerosis
Oxidative stress in endothelial cells contributes to plaque formation in arteries. Humanin reduces reactive oxygen species (ROS) and apoptosis in vascular cells. In ApoE-deficient mice (a standard atherosclerosis model), HNG treatment significantly reduced plaque size and improved endothelial function[12][13].
¶ Insulin Sensitivity
Humanin acts on both the hypothalamus and peripheral tissues to improve insulin sensitivity. It has been shown to lower blood glucose levels in diabetic rats and protect pancreatic beta-cells from destruction[7:1][14].
¶ Role in Longevity
Humanin is increasingly recognized as a biomarker of healthy aging.
¶ Age-Related Decline
Circulating levels of Humanin decrease with age in mice, monkeys, and humans. This decline is associated with the accumulation of mitochondrial dysfunction and age-related diseases[15].
¶ The Centenarian Paradox
Research indicates that exceptionally long-lived individuals (centenarians) and their offspring maintain significantly higher Humanin levels compared to age-matched controls. This suggests that maintaining high Humanin levels may be a protective factor supporting healthspan[15:1].
In C. elegans (nematodes), overexpression of Humanin extends lifespan, confirming a causal role in simpler organisms. In mice, treatment improves healthspan parameters (inflammation, metabolism) but has not yet been proven to extend maximum lifespan significantly[15:2].
¶ Safety Profile and Risks
¶ The "Double-Edged Sword"
The primary mechanism of Humanin—blocking apoptosis—is beneficial for preventing neurodegeneration but poses a theoretical risk in cancer. Apoptosis is the body's natural way of eliminating damaged or cancerous cells.
- Potential Risk: By inhibiting Bax, Humanin could theoretically protect cancer cells from dying, leading to tumor growth or chemotherapy resistance. Some studies indicate Humanin is upregulated in certain tumors (e.g., gastric, pituitary) and may promote chemoresistance in glioblastoma[16][17].
- Protective Context: Conversely, other studies show HNG can protect normal tissues from chemotherapy side effects without reducing the drug's anti-tumor efficacy[18].
Conclusion: Caution is advised. The safety profile is likely context-dependent.
¶ Clinical Status
Currently, Humanin and its analogs (like HNG) remain in the preclinical research stage for therapeutic applications.
- Observational Studies: Human studies have focused on measuring endogenous levels as biomarkers.
- Interventional Trials: There are no FDA-approved Humanin drugs, and no active Phase I/II/III clinical trials testing Humanin administration in humans were found in major registries as of 2024.
¶ References
Hashimoto Y, Niikura T, Tajima H, et al. A novel gene, designated Humanin, suppresses neuronal cell death. Biochem Biophys Res Commun. 2001;283(2):460-468. ↩︎
Moin H, et al. A Review on the Potential Role of Humanin Peptide and its Analogs. Protein & Peptide Letters. 2025. ↩︎
Guo B, Zhai D, Cabezas E, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–461. ↩︎
Morris DL, et al. Humanin induces conformational changes in the apoptosis regulator BAX and sequesters it into fibers. J Biol Chem. 2019. ↩︎
Kim SJ, Guerrero N, Wassef G, et al. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways. Oncotarget. 2016;7(30):46899–46912. ↩︎
Matsuoka M, Hashimoto Y. Mechanisms of action of humanin. Mol Biol Cell. 2013. ↩︎
Kim SJ, et al. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways. Oncotarget. 2016. ↩︎ ↩︎
Romeo M, et al. Humanin Specifically Interacts with Amyloid-β Oligomers and Counteracts Their in vivo Toxicity. J Alzheimers Dis. 2017;57:857. ↩︎
Chai GS, et al. Humanin attenuates Alzheimer-like cognitive deficits and pathological changes induced by amyloid β-peptide in rats. Neurosci Bull. 2014;30:923–935. ↩︎
Niikura T, et al. A Humanin Derivative Reduces Amyloid Beta Accumulation and Ameliorates Memory Deficit in a Transgenic Mouse Model of Alzheimer's Disease. PLoS One. 2011. ↩︎
Xu X, et al. Humanin protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2006;37(10):2613-9. ↩︎
Oh YK, Bachar AR, Zacharias DG, et al. Humanin preserves endothelial function and prevents atherosclerotic plaque progression in hypercholesterolemic ApoE deficient mice. Atherosclerosis. 2011;219(1):65–73. ↩︎
Bachar AR, et al. Humanin is expressed in human vascular walls and protects against oxidized LDL-induced apoptosis. Cardiovasc Res. 2010;88(2):360–366. ↩︎
Conte C, et al. Humanin: A new hope for the treatment of diabetes? World J Diabetes. 2022. ↩︎
Yen K, Mehta HH, Kim SJ, et al. The mitochondrial derived peptide humanin is a regulator of lifespan and healthspan. Aging (Albany NY). 2020;12(12):11185–11199. ↩︎ ↩︎ ↩︎
Asad AS, et al. Humanin boosts chemoresistance in glioblastoma cells. Cancers. 2023;15(16):4061. ↩︎
Ayala MAM, et al. Humanin Promotes Tumor Progression in Experimental Triple Negative Breast Cancer. Sci Rep. 2020;10:8542. ↩︎
Cohen P. The potent humanin analogue (HNG) protects germ cells and leucocytes while enhancing chemotherapy-induced suppression of cancer metastases in male mice. J Natl Cancer Inst. 2014;106(3):dju006. ↩︎